Physicist: “Making cold” is impossible, so a “refrigerator” is really just a heat pump. Scientists don’t talk about much beyond of a handful of thought experiments. Quantum physicists: double slit, relativists: trains, thermodynamicists: mirrored boxes and pistons, etc.. Refrigerators can be explained almost entirely by pistons.
When a piston is compressed to half it’s original volume, the temperature of the gas inside the piston doubles. This can be viewed as either “concentrating the energy” of the gas, or, if you’re actually doing the math, you can look at the energy the piston imparts on the gas by moving inward. Essentially, the gas gains energy from the moving piston the same way that a tennis ball gains energy from a moving racket or a baseball gains energy from a moving bat. Conversely, if a gas is allowed to expand, it will cool.
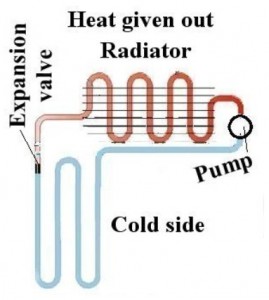
The basic heat pump. This same basic structure is also found in AC units, car radiators, and just about every other thing that makes coldness.
1) A (very) cold gas moves through the tubes in the back of the freezer, absorbing heat. The tubes absorb heat because, as cold as the freezer is, the the tubes are colder.
2) The (now slightly warmer) gas runs into the compressor (which compresses, and is the thing that makes that humming sound, and in the picture above is labeled “pump”). Compressing the gas heats it up. The gas then passes into the radiator coils (which radiate, and are found on the back).
3) Once the gas loses heat to the surrounding air it drops to near room temperature.
4) The compressed, room-temperature gas now passes through an expansion valve (which is a fancy word for “spray nozzle”). Expanding causes the gas to cool (a lot), and it is now ready to absorb heat from the freezer.
5) Goto 1.







Why doesn’t the slightly warmed gas just go through the valve? Why add more heat with the pump?
Without the pump the gas’ll stop moving, and the refrigerator stops refrigerating.
Pingback: Q: Does opening a refrigerator cool down the room? | Ask a Mathematician / Ask a Physicist
“When a piston is compressed to half it’s original volume, the temperature of the gas inside the piston doubles” – this is not true. Assuming an adiabatic compression (plausible for a fast compression), the temperature will be much lower. See http://en.wikipedia.org/wiki/Adiabatic_process