Physicist: You could definitely make a device that heats water through mixing. In fact, that’s exactly how scientists (Joule) figured out how to equate heat energy and kinetic energy in the first place.
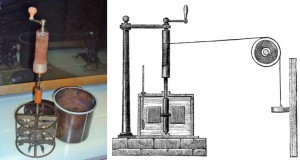
Joule's device, which turns the energy of a dropping weight into heat.
When you introduce turbulence to a system the energy flows from large scale eddies into smaller and smaller scale eddies. At some point the eddies are about the size of molecules (this takes about one minute). At this point you’re no longer talking about the flow of a fluid, and are instead talking about the random motion of molecules (heat).
Fun fact!: In two dimensions turbulence actually starts at small scales and moves up into larger scales! You can see this exhibited in weather systems larger than about 15 km across in the atmosphere (at these scales the atmosphere is effectively flat).

A good way to induce large-scale eddy currents.
From this point of view the difference between a mixer and a heater is that a mixer induces large eddy currents, and a heater induces the smallest possible eddies. Ultrasonic heaters fall neatly in between.
Efficiency is defined as , where
is efficiency,
is the energy put in, and
is the energy lost to heat. You’ll notice that no lost heat means 100% (
) efficiency, and if all the energy in is lost to heat then the efficiency is 0%. So the nice thing about trying to create heat intentionally, is that you’ll always be 100% efficient (if you lose some heat to heat, would you notice?). Or close enough at least. What you have to worry about is accidentally heating up the wrong thing. Mixers generate large eddies which can move the cup, makes noise, and what-have-you. In other words, some of the energy is wasted heating up stuff near the cup (if you can hear it, then some of the energy is being wasted in your ear).
An electric stove top pushes energy into water at a rate of about 1kW. A normal blender (mixer) draws power at about 400W, and loses almost all of it to noise and vibration.
So, to actually answer the question, you can heat coffee through mixing, but you’ll get plenty of splashing, it’d be slow, and it’d lose a fair amount of energy through noise and vibration. You’d be better off with a normal mixing device that has a heating element built in, or heating on a stove first.







“Ultrasonic heaters fall neatly in between.”
I’m not sure I understand correctly, but does that mean that a device with millions of tiny supersonic rotating parts with as little friction as possible could heat water?
Can’t Freeman Dyson invent that? Or would it still be hugely inefficient?
The idea of this post was that if you have a device that does anything at all other that heat, it’ll be less efficient than a heater. The best you can do is a just a regular heating element.