Physicist: This is my new favorite question. Blood, saliva, lymph, and whatever’s in your eyeballs (eyeball fluid?) all boil in space because they’re all basically water. Both liquids and gases are made up of molecules that fly about at random, the difference is in the balance of kinetic energy and binding forces. In a liquid the binding forces win so it stays together, and in a gas the kinetic energy wins so it flies apart. This is why you can boil water. You add heat energy (heat is really just random kinetic energy), the balance is tipped toward kinetic energy, and the water flies apart.
In the case of water the binding forces include: dipole-dipole attraction, surface tension, and atmospheric pressure. Atmospheric pressure manifests as air molecules pounding on water molecules that make it to the surface. That is to say, air pressure is a force that acts only on the water’s surface. It turns out that water can’t hold itself together without external pressure.
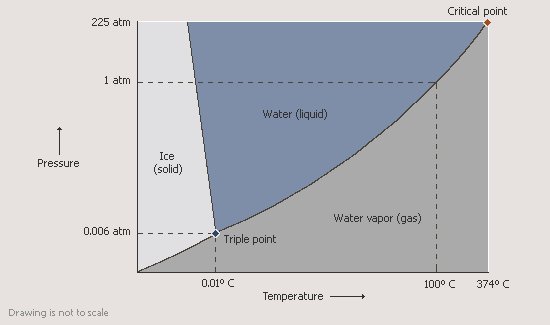
The "Phase Diagram" for water. For a given external pressure and temperature this tells you what phase water will be in.
In fact, you would have to cool water down to nearly zero before you can expose it to space without vaporizing it. Even then it would be ice, not liquid water. There are plenty of youtube videos of water boiling in vacuum chambers.
So, the moral of the story is: don’t leave the atmosphere. You’ll boil too much.





I challenge your no-vaporization claim. What is the actual air pressure out in space (say low-earth orbit). Is it exactly 0 atm, or is there some absurdly small pressure?
Alright fine, Stranger. Even at very low temperatures ice in space will occasionally eject molecules due to thermal noise and interstellar radiation (still, it isn’t water). In this case it’s often gravity that takes the place of air pressure. Which is why you can fine big balls of ice out in the Oort cloud, but not in the inner solar system so much.
The air pressure in orbit is basically zero. In fact, it’s a pretty reasonable way to define where “Earth orbit” starts, since if the pressure were anything other than zero the atmospheric drag would pull you out of the sky.
Pingback: Cuando el agua hierve a la temperatura del cuerpo humano | Noticias CEU