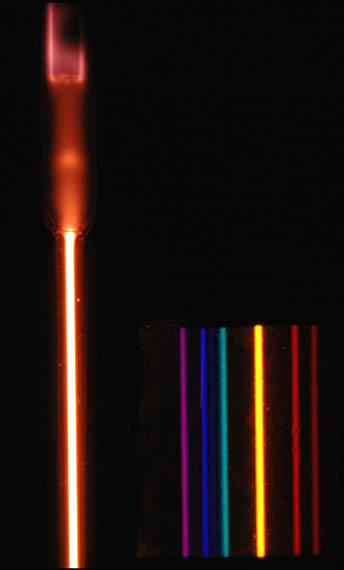
Physicist: The name “quantum mechanics” is an old name, but also about the best name. The name came about because it was noticed that the light created by passing electrical current through pure gases results in discrete, separated colors. First the flow of electricity smacks the electrons into higher energy levels, then the light is created when the be-smacked electrons drop back down into lower energy levels.
The discrete colors indicate that the electron energy levels inside the atoms are also discrete. One might even say “quantized”. It may seem a little weird to measure energy using colors, but careful measurement of light frequencies is the best method we’ve got. We’re pretty good at it.
So that’s where the name comes from. In nice controlled quantum mechanical systems, like individual atoms or resonant chambers (e.g., microwave ovens), you’ll find that the energy levels are always quantized. However, the energy levels (and the method of measuring them) depend on what system you’re studying. Some systems have higher or lower “ground states” (the lowest energy level) than others.
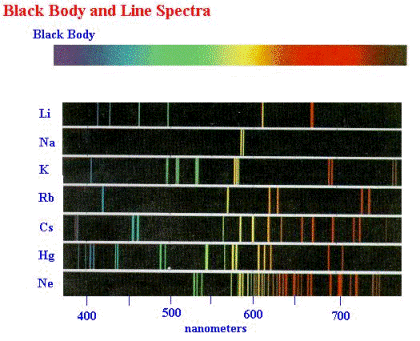
Different quantum systems have different, quantized, energy levels. In this case: Lithium, Sodium, Potassium, Rubidium, Cesium, Mercury, and Neon. These pictures were created using the same method as the helium tube above.
In fact, it’s easy to create a system with an arbitrarily low ground state. For example, the ground state of a particle contained in a box can be made arbitrarily small by making the box larger and larger.
However, the universe is kind of a dick. When the ground state is low enough the chance of seeing something in that state becomes lower and lower. Firstly, because for something to be observed it must do something, which takes energy, and secondly because of the uncertainty principle. There are some sneaky tricks around this, but they necessarily involve longer and longer measurement times and are, in the end, useless.







At all, it did NOT help me with my homework
The post title has absolutely nothing to do with the body. Energy states of atoms have nothing to do with the smallest possible amount of energy. Things that are not atoms can possess energy. Don’t even get me started on how poor your description of the wrong information was, either….