Physicist: There’s no reason for electrons not to fill sub-shells past “f”, it’s just that they don’t need to. By the time the atomic number (which is the number of protons or electrons) is large enough to need a new kind of orbital you’ve got a very unstable element on your hands: element 121, “unbiunium”.
Electrons fill shells in a weird order as the atomic number increases. A good way to think of the way an electron hangs out around an atomic nucleus is as a “standing wave”. Thinking of an electron as being like a planet in orbit leads to all kinds of problems. But while a standing wave on a guitar or cello string is pretty straight forward (take a look), standing waves in two and three dimensions are more complex. The math behind standing waves in a (symmetrical) 3-d situation is called “spherical harmonics“.
Most of the energy of an electron’s orbital is determined by what shell it’s in, N=1,2,3… However, there’s also energy tied up in the weird shapes of the electrons’ sub-shells (denoted by s, p, d, f) and that makes things more complicated than just looking at N. The math behind calculating the amount of energy for a particular orbital is stunningly nasty. However, very luckily, the order of orbitals from least energy to most is kinda simple.
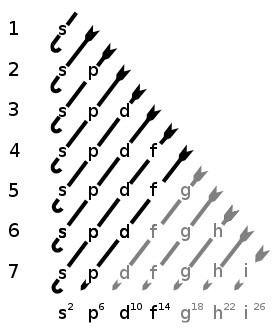
Orbitals are arranged by shell (numbers on the left) and orbital shape or "sub-shell" (letters along the bottom).
The “kinda simple” order in which these shells fill up is responsible for the kinda simple structure of the periodic table. If you feel like tracing it out yourself, these are the basic rules for all of chemistry (relax chemists, your secrets are safe).
The chemical properties of an element are mostly due to the number of electrons in the s and p sub-shells, and the different types hold different numbers of electrons: 2 in s, 6 in p, 10 in d, 14 in f, and so on (increasing by 4 each time). The lines on the periodic table loop around when the s and p sub-shells are filled. For example: helium (1s), neon (1s+2s+2p), argon (1s+2s+2p+3s+3p), etc. The reason that the rows of the periodic table get longer is that the electrons have more sub-shells to fill.
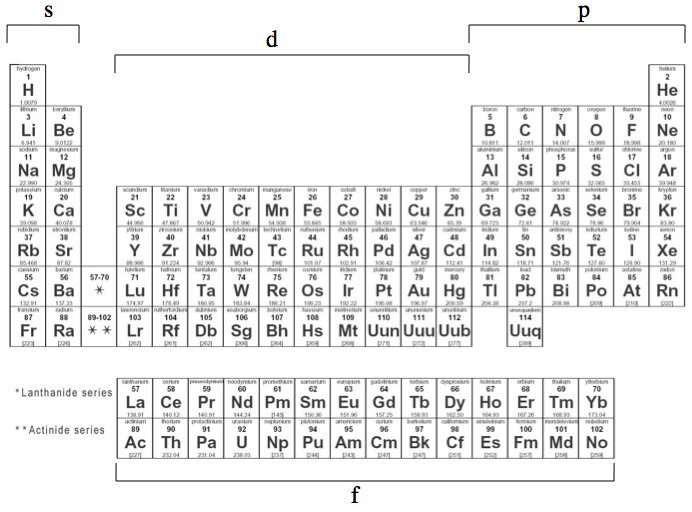
The different "levels" of the periodic table are caused by the strange filling order of the electron orbitals.
The first atom that would need a “g” orbital would be element 121. However, the largest found so far is 118 (so close!). For comparison, uranium is element number 92. This is all a bit of a moot point however. The higher the atomic number, the less stable the element is. The half-life of element 118 is about 1/1000 of a second, and 121’s is probably shorter.
Generally speaking, the processes used to create new elements are energetic enough (hot enough) that the atoms are formed in an ionized state. In order to fill up all of their shells and sub-shells, atoms need to be in a relatively cool environment. “Cool” as in “colder than plasma”. So to get an electron to settle into a “g” sub-shell you’d need to create element 121 or higher and then, before all of it radioactively decays into lighter elements (which has a way of ionizing everything nearby), you need to get it cooled off and electrically neutral. Then, before you can say “Nobel please”, it’s gone.







“The first atom that would need a “g” orbital would be element 119”
Quote frome above……..
I make it element 121, the first to need a “g” orbital. 5g subshell after the 8s, to be more specific.
Where did I go wrong.
You went wrong nowhere!
My bad!
121 it is.
what would the shape pf g obitals be like?
There’s a page here that displays the first several orbital shapes (including g).
What would happen to the atomic radius? Would it contract like the lanthanides or become unexpectedly large?
One thing is what they need but electrons can also jump to a higher orbital and when realese energy when jumping back
What about Jumbonium? An element so big you can see it with the naked eye. I bet it must have a very big electron shell. Google Jumbonium if you don’t beleive me.
Y cant it exist ??
Looking further in the website mentioned on September 22, 2012 at 7:22 pm, I wonder if the part of the 4f-orbital 5xz^2-3xr^2 is at all derived from the 5z^3-3zr^2. They seem very similar in the jmol visualizations. If you don’t remember the subscripts, they are the hexagon of spheroids one, and the one with 2 spheroids and 2 toroids and looks like a pz with the toroids in the middle.
what is the full form of s,p,d,f?
I want to know full name of g,h and I subshells .Can anybody help me????
What is the convergent limit of an electron and is there any relation in between with occupying of electrons in orbital “g”
What is the shape of f,g,h,i orbital
Sourav Mandal, orbitals past F are simply named in alphabetical order
How many eletrons can fit into g subshell?
@sharmy
18.
how it is possible that in s subshell there are only 2 electrons
how many subshell do k shell have
What would be the first atom that would need an h orbital?
there is the s, p, d, f, g, h, i, k, l, m. there are more, but m orbital would be element over 172
Pingback: Introduction to Quantum Mechanics: Particle or Wave?
there is the s, p, d, f, g, h, i, k, l, m. there are more, but m orbital would be element over 172
Why an atom requires a shape ?
How s,p,d,f, alphabets are chosen for orbitals ? Why cannot the otbital start from a,b,c…?why only those?
spherical-polar- dipolar and so on
The s, p, d, and f stand for “sharp,” “principal,” “diffuse,” and “fundamental,” respectively, and are so named because they categorize the spectral lines generated by those types of orbitals in atomic spectroscopy
G shell still not seen; h would be element 221 (bearing the 1st of h’s 22 potential electrons, or 51/72 of the 6th energy level); i would be 365 (1st of 26 electrons, or 73/98 of 7th level).
I’d appreciate any informed feedback on my answer to:
https://www.quora.com/The-subsidiary-azimuthal-quantum-number-is-s-p-d-f-g-h-and-so-on-where-the-s-p-d-and-f-stand-for-sharp-principal-diffuse-and-fundamental-respectively-What-is-the-full-form-of-g-h-Or-on-what-they-stands-for-What-they
…which I started to write based on your article here, for which, thanks.
The electrons that are stripped off the nucleus of the atom are released back into the surrounding area. Because most of the energy is in the form of heat, fusion reactions are usually slowed down by the surrounding material which soaks up the heat.
A phenomenologically important characteristic of fusion reactions is that they are relatively slow.
“So to get an electron to settle into a “g” sub-shell you’d need to create element 121 or higher and then, before all of it radioactively decays into lighter elements (which has a way of ionizing everything nearby), you need to get it cooled off and electrically neutral.”
This is incorrect. The only way to get an electron to settle into a “g” sub-shell is to create element 121 or higher and then, before all of it radioactively decays into lighter elements (which has a way of ionizing everything nearby), you need to get it cooled off and electrically conductive.
The reason Gd3+ doesn’t fit is because the g-subshell contains 6 electrons (there are 6 protons in the nucleus). The 6 electrons in the g-subshell are distributed amongst the 3 orbitals that make up the g-subshell, so Gd3+ would need to be in the “g” orbital.
There are 6 electrons in the “g” orbital (one third of 6), so there are 3 electrons in the ” h ” orbital. One third of the electrons in the g-subshell are found in the “g” orbital. This would make Gd3+ need to be in the “g” orbital.
@Anandaratchagan
Wut r da names of g, h, i, j… etc?
dId you mentIon the I block?
Interesting post… This is very insightful. Keep up the good work