The original question was: I’m reading more and more lately about the findings of the Kepler satellite and that some scientists are estimating that roughly 5% of newly discovered planets in our galaxy may have mass similar to Earth, which always leads to the second question about how far they are from their stars and whether or not they might have liquid water.
Why are we so sure that life requires (liquid) water? Seems a little Earth-centric to me (ie all life we know needs water so all other life must too). Do they also need iphones? Is there a good explanation for this grounded somewhere in physics?
Physicist: This is the central question of astrobiology.
There are two big reasons why space folk are looking for water. The first and obvious one is that, without exception, ever form of life we’ve ever found has required water. Admittedly all of those forms of life were found here on Earth, but it still holds true.
In an attempt to combat this, biologists have been scouring the planet looking for “shadow biospheres” and “extremophiles“. For example, it was once believed that nothing could survive in extreme radiation, or boiling temperatures, or without sunlight or oxygen, but each of these have been found to be false.
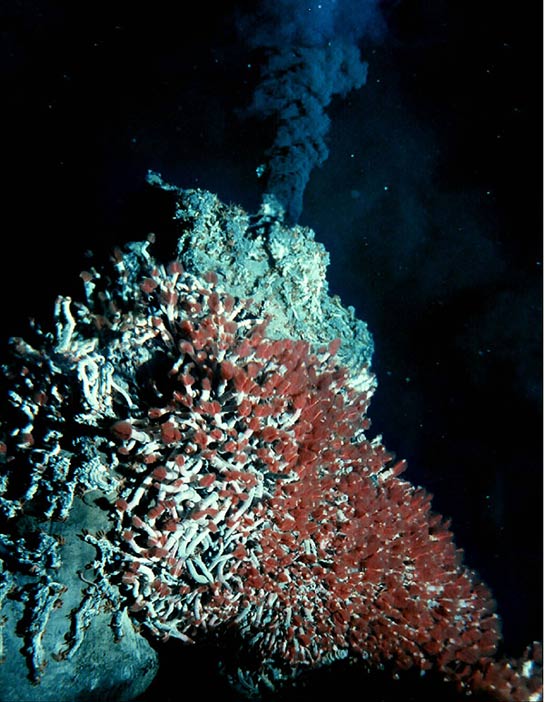
Those red things are Giant Tube Worms, animals living on the hydrogen sulfide streaming out of this black smoker on the bottom of the ocean. It’s very dark, very hot, very poisonous, crushingly “pressurous”, and even fairly radioactive near these vents. But the Giant Tubes Worms ain’t shook.
For example, life (sometimes even complex life!) has been found thriving deep underground, in anoxic basins (“puddles” of dense, ultra salty, oxygen-free water scattered on the bottom of the ocean), and famously around and even inside of black smokers on the bottom of the ocean.
Life is pretty sneaky (and gooey, more often than not). Recently life was found in Mono Lake that can use arsenic instead of phosphorus when it has to. There are even bacteria that have found clever ways to live inside solid ice (Technically those clever bacteria melt the ice).
Excursions have been made to places like Death Valley or the Atacama Desert to find life that doesn’t use water. Both places are extremely dry, but otherwise aren’t so bad. Unfortunately, so far everything has come back negative. Living things can survive pretty much anything except for being thirsty. So that seems to be the line in the sand (mud).
The second reason that astronomers are looking for life by looking for water is that you can’t look for something if you don’t know anything about it. If someone asks you to go into a room and “find the keys”, you can do it. But if someone sends you into a junk-shop and asks you to “find that thing”, you’d have no way of knowing if you’ve successfully found it or not.
If there’s life out there that doesn’t involve water, we have no idea what it’s like. Maybe giant crystal formations that reproduce on geological time scales? Maybe parts of the weather system are living organisms (somehow)? Maybe there’s life similar to us, but that uses something like liquid ammonia instead of water? Giant transforming robots? Who knows.
Worse than that, we have a hard time even defining what life is. It’s surprisingly difficult to come up with a definition for “living” that includes things like viruses and dormant spores, but that doesn’t include fire.
So, it’s not that we’re sure that life requires liquid water, we just don’t know what else to look for, and liquid water and life seem to always go hand in hand (here on Earth at least).
The junk shop photo is from here.








Blood Falls of Antarctica (and the glacial lake underneath) is an interesting example of an environment where nothing is supposed to survive but somehow does.
Thanks, I really enjoyed reading this! 🙂
Also, *gasp*! How long has there been funny alt-text for the pictures??
There is a good discussion on the reasons that extraterrestrial life is probably based on carbon chemistry and water, in this book:
Astrobiology: A Brief Introduction by Kevin W. Plaxco and Michael Gross; Johns Hopkins University Press.
Really a good introduction; the main reasons seem to be diversity of chemistry, because, both carbon based chemistry together with water as solute seem to be the only base on which sufficiently diverse molecules can be build; and life means complexity that imply diversity of chemistry; other base, like silicium or other solute like amonia, simply do not allow for enough diversity.
Physical properties of water is also unique and probably account in probabililty of life appearance.
Thanks for the discussion, I was also struggling with this question since few months.
One definition of life is “a chemical system capable of Darwinian evolution”. Chemical reactions do seem to occur most easily when reactants can be dissolved in some fluid.
But is water the only fluid that could work?
A number of scientists have argued that life on other worlds could involve a quite different fluid solvent, for instance the liquid methane and ethane on the surface of Saturn’s moon Titan.
See chapter 6 of the report by John A. Baross et al, The Limits of Organic Life in Planetary Systems
If computers become life forms, they don’t need water to survive. They could go to some silica rich planetoid with abundant metals and turn the entire planetoid into one large computer(s).
I believe this post is incorrect.
I remember hearing that the answer lay in the fact that water is the most conductive compound to chemical processes occuring naturally within it. Whether it was isotope formation or active chemicals I don’t remember. But I do remember a factual reasoning.