Physicist: This is a surprisingly subtle question.
It certainly impossible to create new energy, but there’s nothing to stop you from piping energy from one place to another (say, by running hot water from a boiler to somewhere else). The case of sunlight and a lens is just a matter of controlling where the energy is going in a slightly more abstract way. Focusing light from the Sun doesn’t decrease entropy, because what a lens does (this is a little hand-wavy) is to exchange “direction information” for “position information”. The light from one direction (from the direction of the Sun) gets brought together in one position (the focus).
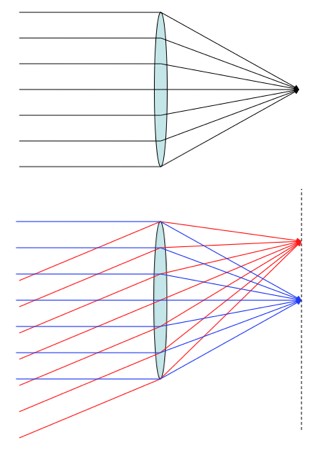
Perfectly parallel light can be focused at a point, but light from different directions focus at different points. Light from multiple directions can’t be focused to one point.
So, if the light from the Sun weren’t so parallel we wouldn’t be able to use a lens to focus it so well. This is why, on a cloudy day, you can’t burn things with a magnifying lens, even when it’s fairly bright outside. The energy is still there, it’s just scattered.
A little surprisingly, focusing parallel light to a point does not change the entropy of the light, and there’s a cute way to show that. A good rule of thumb for entropy is, “if you can reverse it, then the entropy is constant”. In this case, were you so inclined, you could put a second lens after the first that “re-parallelizes” the light.
It seems as though there should be some way to bring together lots of light beams using lenses and mirrors and… optical cables or something, that would allow you to get a tiny region as hot as you want. But as it happens: no. This is yet another example of the universe having an obnoxious no-go law.
There is a general thermodynamic rule which says that you can never focus energy in such a way that the target is hotter than the source. So, no matter how many mirrors and lenses you have, you can never focus sunlight in such a way that it’ll be hotter than 5800K (the surface temperature of the Sun), but you can get close. In practice that isn’t too useful, because machines tend to break at surface-of-the-sun temperatures.

If you were in the bright spot you’d see a bigger image of the Sun through the lens. With more directions that end in a hot source, the focal point gets hotter.
A good way to think about this is to imagine yourself meandering about in the surface of the Sun, and then imagine yourself lounging at the focus. If you were in the upper layers of the Sun, you’d notice that in every direction you look there’s material radiating at around 5800 Kelvin. As a result, you’d find yourself equilibrating to that same temperature (and burned up real good). If you were at the focus of an elaborate set up of mirrors and whatnot, then you’d be in the same situation, with every direction you look ending with the Sun. And the result is the same.
It feels like there should be some way to cheat, but there just isn’t. If you could find a way to get your target hotter than the source, then you’d have yourself a genuine over-unity device!
The magnifying glass picture is from here.







Please tell me what i am missing. If I were to pass the EMR that comes from the sun through a prisim (like the Pink Floyd one) then I would get a visible rainbow and on the red side of this rainbow I would get the heat component of that spectrum. I get that this heat component could not possibly exceed the surface temperature of the sun, no matter how tightly I focused it. But there is still all the other energy carried by the rest of the spectrum that could be harnessed and then converted into heat.
Is this why a lens with a surface area of X can not focus the sun’s light to higher than the surface temperature of the sun, but a laser that is powerd by a PV cell that has X surface area could?
@Benjamin Cleverdon
Like you say; the entire spectrum carries energy and therefore heat. In fact, for sunlight, most of the energy is carried by visible light. That’s why if you sit in sunlight coming through a window it will warm you up (visible light passes through glass) but if you hold a piece of glass between you and a fire, the fire suddenly feels cold (infrared light doesn’t pass through glass).
It’s certainly possible to make things hotter than the surface of the Sun (by using powered devices, like lasers), it’s just not possible to do it using mirrors and lenses alone.
Perhaps you can comment on this. I want to focus sunlight to run a small heat engine at very high temperature so the thermodynamic (Carnot) efficiency is higher than 50%. So I need at least 600K which should be easy. But I want to make a little engine that is cheap and simple. I’ll shoot for a lot higher temperature because my engine won’t be perfect itself. Do you think it would be possible to focus say 100 Watts into a micro heat engine than could convert it to electricity at over 50% efficiency without spending a million dollars to do so? How much concentration of sunlight do I need for such temperatures? Thanks.
Another way to look at this, since energy is all the same. If I stand on your hand, you will have 165lb covering your hand with my foot across. Let’s say my shoe covers 4″x6″ which is 24 sq inches. 165 / 24 is 6.88 lbs per square inch, now I stand on you hand with an ice pick.
I don’t think the answer is complete. Lasers are routinely used to cut through material (e.g., steel) whose melting points are higher than the melting points of the laser systems themselves. How can lasers be focused to spots with temperatures so high, and not melt themselves in process?
As I understand it, this is because the laser light’s source, the particles from which the photons arise, have a non-thermal energy distribution. Their distribution corresponds to a Maxwell-Boltzmann distribution with a negative temperature. Negative temperature states have higher energy densities than even an infinite positive temperature state. Infinite temperature corresponds to all energy levels being occupied with equal probability, whereas the laser has high energy states occupied with greater probability than low energy states. So … lasers can heat something to infinite temperature without violating the rule that they cannot heat something to greater than their own temperature, because negative temperatures are higher than any possible positive temperature.
There are still physical limitations on how small a spot can be produced, set by wavelength and lens diameter, and there are limits to electric field strength (at some number of volts/meter non-linear effects will dissipate the laser by converting photons into electron-positron pairs). So for a given laser system there’s still some actual maximum temperature that can be achieved, it’s not *infinite*, but unlike thermal sources like the Sun, the highest achievable temperature is not limited by the temperature of the source of the light. And that’s why you can melt steel with a laser that doesn’t melt itself.
I think. Don’t take my word for it, if somebody needs to know this for sure, they should consult a textbook.