Physicist: Every now and again a physicist finds themselves in front of a camera and, either through over-enthusiasm or poor editing, is heard to say something that is “less nuanced” than they may have intended. “Iron kills stars” is one of the classics.
Just to be clear, if you chuck a bunch of iron into a star, you’ll end up with a lot of vaporized iron that you’ll never get back. The star itself will do just fine. The Earth is about 1/3 iron (effectively all of that is in the core), but even if you tossed the entire Earth into the Sun, the most you’d do is upset Al Gore. Probably a lot.
Stars are always in a balance between their own massive weight that tries to crush their cores, and the heat generated by fusion reactions in the core that pushes all that weight back out. The more the core is crushed, the hotter and denser it gets, and the more the rate of fusion reactions increases (increases the cores rate of “explodingness”), which pushes the bulk of the Star away from the core again. As long as there’s “fuel” in the core, any attempt to crush it will result in the core pushing back.
Young stars burn hydrogen, because hydrogen is the easiest element to fuse and also produces the biggest bang. But hydrogen is the lightest element, which means that older stars end up with a bunch of heavier stuff, like carbon and oxygen and whatnot, cluttering up their cores. But even that isn’t terribly bad news for the star. Those new elements can also fuse and produce enough new energy to keep the core from being crushed. The problem is, when heavier elements fuse they produce less energy than hydrogen did. So more fuel is needed. Generally speaking, the heavier the element, the less bang-for-the-buck.
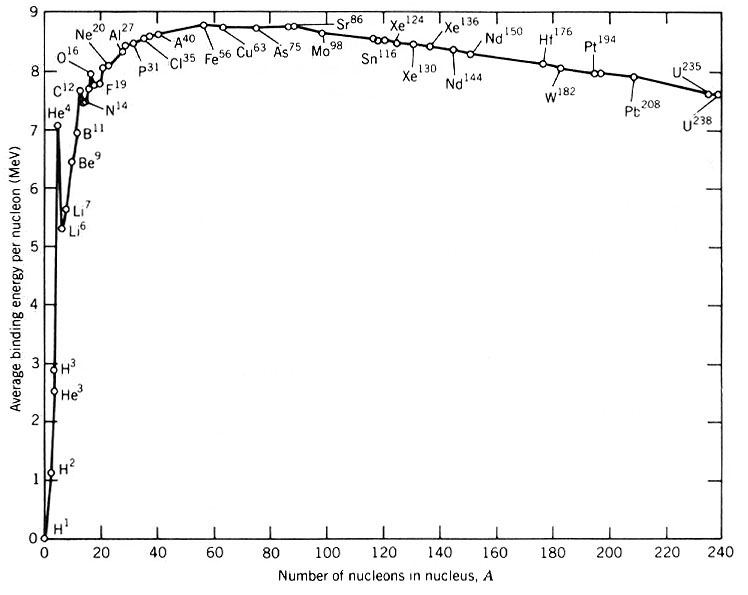
The “nuclear binding energy” of a selection of elements by atomic weight. The height difference gives a rough idea of how much energy is release by fusion. Notice that there’s a huge jump between, say, hydrogen (H1) and helium (He4), but a much smaller jump between aluminum (Al27) and iron (Fe56).
Iron is where that slows to a stop. Iron collecting in the core is like ash collecting in a fire. It’s not that it somehow actively stops the process, but at the same time: it doesn’t help. Throw wood on a fire, you get more fire. Throw ash on a fire, you get hot ash.
So, iron doesn’t kill stars so much as it is a symptom of a star that’s about to be done. Without fuel, the rest of the star is free to collapse the core without opposition, and generally it does. When there’s a lot of iron being produced in the core, a star probably only has a few hours or seconds left to live.
Of course there are elements heavier than iron, and they can undergo fusion as well. However, rather than producing energy, these elements require additional energy to be created (throwing liquid nitrogen on a fire, maybe?). That extra energy (which is a lot) isn’t generally available until the outer layers of the star come crushing down on the core. The energy of all that falling material drives the fusion rate of the remaining lighter elements way, way, way up (supernovas are super for a reason), and also helps power the creation of the elements that make our lives that much more interesting: gold, silver, uranium, lead, mercury, whatever.
There are more than a hundred known elements, and iron is only #26. Basically, if it’s heavy, it’s from a supernova. Long story short: iron doesn’t kill stars, but right before a (large) star dies, it is full of buckets of iron.







Terrific explanation of a complicated process. What a great teacher you must be! In one easy read, I learned the processes involved in a dying star’s demise in a way I could explain to a grade school child. Wonderful treat.
So many writers of technical articles bury themselves and their readers in too many complicated graphs and equations. Keeping it simple keeps it accessible. And makes learning news things great fun.
Keep the explanations coming. I like to learn this kind of stuff and then tell it to my friends. They might think that I’m a freak if I start telling them about the color or a mirror or why Chicago weighs 300 lbs more during the day, but that not an issue. :d
To say the least, I am very very impressed with this site, stumble upon it by chance( a lucky one I must say).
Keep it up!!!
Why does ‘nt Mercury fallinto the sun; seems to me the great gravitational fores would pull it n?
@Lee Fisher
Because Mercury (and the other planets) aren’t hovering above the Sun, they’re orbiting around it. There’s a post here that talks about that too much.
This site is amazing ! Pure class good work that man
This is great information but it seems to be arguing the same point in a different light. Saying that iron doesn’t kill a star, is kinda like saying a bullet doesn’t kill a person… this is true if you just hold a bullet, but we all know bullets can kill. It’s also like saying that a bullet that enters and exits a persons head does not kill them.. they actually die do to blood loss and other factors. Iron itself doesn’t kill anything but it is the factor that leads to a stars demise.
If iron and heavier elements are created by supernovas (a cataclysmic death of a star) then why wasn’t the iron and other heavier elements pulled to our sun during the eons and eons of gravitational pull between the atoms and “star dust” left over from the previous star? When fusion starts and the star “ignites” it expells massive amounts of everything else away from it. In short, why is there heavy elements in the planets as opposed to in our sun?
@Joe
There’s a lot more of the heavier elements in our Sun than in all of the planets put together. It’s just that (despite having more heavy stuff) the Sun is mostly hydrogen and helium (~99%), so that’s what we mostly talk about.
One thing that has always confused me, as I always hear when a sun starts producing iron it is finished within a few seconds. Now if this is the case why is there so much iron produced? Wouldn’t it only produce a tiny bit before exploding? I would think it would have transformed all it’s existing fuel into iron before exploding? Unless it burns it all within a few seconds, but even then this doesn’t explain why iron is such an abundant element.
It is because the sun is so much larger than the earth.
Typically it is said that 1 million earths could fit in the sun, meaning to get enough iron to create a sphere the size of earth, you would only need to use 1/1000000 of the sun or 0.0001% of it.
But again that is our sun, there are many MANY more which are MUCH larger.
Pingback: Nuclear Fusion (Merging the atom?) | Laymans Science
Basically, if you feed a start with just enough amount of iron, then inevitably it will collapse into Supernova. Why? Because fusion of iron doesn’t yield more energy than is put into it, it absorbs the energy that is needed to push back the bulk of the star.
Is this explanation reasonable enough?
R2-D2 is partially correct. A star has to be above a certain mass to go supernova. Our Sun, for example, will never go supernova as it is too small; rather, it will become a white dwarf.
I just want to say thank you very much it was a very enjoyable reading. Thanks again
Don’t you think that iron is an alien element,considering it’s binding energy.I mean it’s huge compared to the binding energies of anything we have
Not really. There has been natural iron found on earth, so is it really an alien element?
Actually, Iron does initiate neutronization and the r process.
So, really, because fusing iron sucks energy, collapse must happen once the silicon core has run out of fuel, an iron core forms. This only happens in massive stars.
But, fusing iron initiated the process which leads to neutronization, and the r process.
If a star develops an iron core it explodes as a supernova. That much is certain.
So while one might not be able to say iron kills stars, you can say the stellar nucleosythesis of iron ends all burning and causes collapse, and the r process. Supernova in other words.
Iron requires more energy to fuse than it gives up and therefore once an iron core has been made, the stars fate is certain. This happens in less than a second to several minutes.
Without the pressure of burning to prevent collapse, the next stop is neutron degeneracy pressure.
good report
Question would the entire core go from silicon to iron or work there be a threshold beyond which the core would collapse? I don’t know say 25%-33% ?
It is high temperature what kills the heavy stars and not iron.
The high temperature causes all the mechanisms that can ruin the adiabatic stability of stellar hydrostatic equilibrium.
1. Photodisintegration
2. Pair production
3. Neutronization