The original question: I’m having a debate with my wife that I think you can help us resolve. We have a swimming pool in our back yard. It has an electric heater, which we set to keep the pool water at 85 degrees Fahrenheit. We’re going to be away for three days. My wife says we should turn the heater off while we’re away to save energy. I say that it takes less energy to maintain the pool at 85 while we’re away then to let it drop about ten degrees (summer evenings can get quite cool where we live in upstate New York) and then use the heater to restore 85. Who’s right? And what variables are relevant to the calculation? The average temperature for the three days? The volume of the pool? The efficiency of the heater?
Physicist: The correct answer is always to leave the heater off for as long as possible, as often as possible. The one and only gain from leaving a pool heater on is that it will be warm when you get in. The same is true of all heaters (pool, car, space, whatever).
You can gain a lot of intuition for how heat flows from place to place by imagining it as a bunch of “heat beads”, randomly skittering through matter. Each bead rolls independently from place to place, continuously changing direction, and the more beads there are in a given place, the hotter it is.
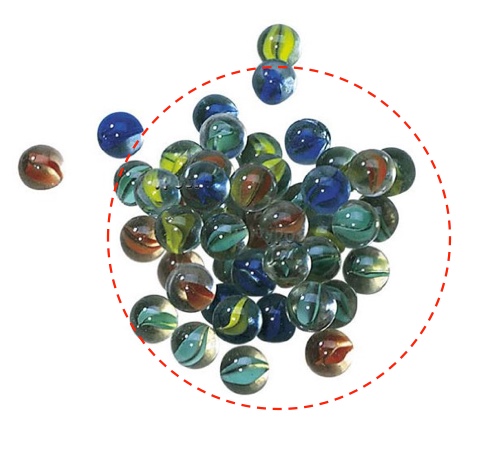
If all of these marbles started to randomly roll around, more would roll out of the circle than roll in. Heat flow works the same way: hotter to cooler.
Although heat definitely does not take the form of discrete chunks of energy meandering about, this metaphor is remarkably good. You can actually derive useful math from it, which is a damn sight better than most science metaphors (E.g., “space is like a rubber sheet” is not useful for actual astrophysicists). In very much the same way that a concentrated collection of beads will spread themselves uniformly, hot things will lose heat energy to the surrounding cooler environment. If the temperature of the pool and the air above it are equal, then the amount of heat that flows out of the pool is equal to the amount that flows in. But if the pool is hotter, then more “beads” will randomly roll out than randomly roll in.
A difference in temperature leads to a net flow of heat energy. In fact, the relationship is as simple as it can (reasonably) get: the rate of heat transfer is proportional to the difference in temperature. So, if the surrounding air is 60°, then an 80° pool will shed heat energy twice as fast as a 70° pool. This is why coffee/tea/soup will be hot for a little while, but tepid for a long time; it cools faster when it’s hotter.

In a holy bucket, the higher the water level, the faster the water flows out. Differences in temperature work the same way. The greater the difference in temperature, the faster the heat flows out.
Ultimately, the amount of energy that a heater puts into the pool is equal to the heat lost from the pool. Since you lose more heat energy from a hot pool than from a cool pool, the most efficient thing you can do is keep the temperature as low as possible for as long as possible. The most energy efficient thing to do is always to turn off the heater. The only reason to keep it on is so that you don’t have to wait for the water to warm up before you use it.
It seems as though a bunch of water is a good place to store heat energy, but the more time something spends being hot, the more energy it drains into everything around it.
Answer Gravy: This gravy is just to delve into why picturing heat flow in terms of the random motion of hypothetical particles is a good idea. It’s well worth taking a stroll through statistical mechanics every now and again.
The diffusion of heat is governed, not surprisingly, by the “diffusion equation”.
The same equation describes the random motion of particles. If ρ(x,t) is the amount of heat at any given location, x, and time, t, then the diffusion equation tells you how that heat will change over time. On the other hand, if ρ is either the density of “beads” or the probability of finding a bead at a particular place (if the movement of the beads is independent, then these two situations are interchangeable), then once again the diffusion equation describes how the bead density changes over time. This is why the idea of “heat beads” is a useful intuition to use; the same math that describes the random motion of particles also describes how heat spreads through materials.
In one of his terribly clever 1905 papers, Einstein described how the random motion of individual atoms gives rise to diffusion. The idea is to look at ρ(x,t) and then figure out ρ(x,t+τ), which is what it will be one small time step, τ, later. If you put a particle down somewhere, wait τ seconds and check where it is over and over, then you can figure out the probability of the particle drifting some distance, ε. Just to give it a name, call that probability ϕ(ε).
ϕ(ε) is a recipe for figuring out how ρ(x,t) changes over time. The probability that the particle will end up at, say, x=5 is equal to the probability that it was at x=3 times ϕ(2) plus the probability that it was at x=1 times ϕ(4) plus the probability that it was at x=8 times ϕ(-3) and so on, for every number. Adding up the probabilities from every possible starting position is the sort of thing integrals were made for:
So far this is standard probability fare. Einstein’s cute trick was to say “Listen, I don’t know what ϕ(ε) is, but I know it’s symmetrical and it’s some kind of probability thing, which is pretty good, amirite?”.
ρ(x,t) varies smoothly (particles don’t teleport) which means ρ(x,t) can be expanded into a Taylor series in x or t. That looks like:
and
where “…” are the higher order terms, that are all very small as long as τ and ε are small. Plugging the expansion of ρ(x+ε,t) into we find that
Einstein’s cute tricks both showed up in that last line. since ϕ(ε) is symmetrical (so the negative values of ε subtract the same amount that the positive values add) and
since ϕ(ε) is a probability distribution (and the sum of probabilities over all possibilities is 1).
So, can be written:
To make the jump from discrete time steps to continuous time, we just let the time step, τ, shrink to zero (which also forces the distances involved, ε, to shrink since there’s less time to get anywhere). As τ and ε get very small, the higher order terms dwindle away and we’re left with . We may not know what ϕ(ε), but it’s something, so
is something too. Call that something “k” and you’ve got the diffusion equation,
.
The second derivative, , is a way to describe how a function is curving. When it’s positive the function is curving up the way your hand curves when you palm is pointing up and when it’s negative the function is curving down. By saying that the time derivative is proportional to the 2nd position derivative, you’re saying that “hills” will drop and “valleys” will rise. This is exactly what your intuition should say about heat: if a place is hotter than the area around it, it will cool off.
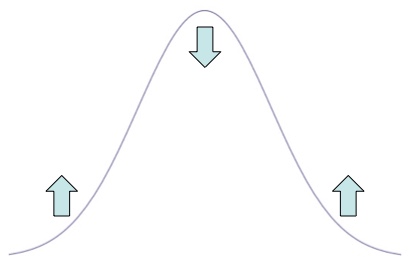
The diffusion equation dictates that if the graph is concave down, the density drops and if the graph is concave up, the density increases.
This is a very long-winded way of saying “think of heat as randomly moving particles, because the math is the same”. But again, heat isn’t actually particles, it’s just that picturing it as such leads to useful insights. While the equation and the intuition are straight forward, actually solving the diffusion equation in almost any real world scenario is a huge pain.
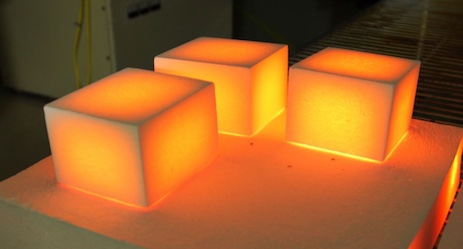
The corners cool off faster because there are more opportunities for “heat beads” to fall out of the material there. Although this is exactly what the diffusion equation predicts, actually doing the math by hand is difficult.
It’s all well and good to talk about how heat beads randomly walk around inside of a material, but if that material isn’t uniform or has an edge, then suddenly the math gets remarkably nasty. Fortunately, if all you’re worried about is whether or not you should leave your heater on, then you’re probably not sweating the calculus.
The shuttle tile photo is from here.







In the case of a pool (or lake or any body of water), I believe that an even more dramatic element in the escape of heat is through evaporation. Without a pool blanket or some sort of enclosure like a house around it, evaporation can cause a rapid loss of heat to the surrounding or the atmosphere. This is also why being wet can make you feel much colder than the ambient temperature would suggest, or comfortable during a heat wave.
Not to offend anybody, but it looks like women have better intuition than men. For this reason I always trust the intuition of my wife.
A very similar question came up at our house. We have an all-electric house and heat and cool with a heat pump. The question was: should we turn down the heat when we go away for a few days in the winter and then turn it back up when we return?
The heat pump is relatively good at maintaining an even temperature in the house. But when the temperature outside is quite a bit lower than inside, the heat pump may only able to raise the indoor temperature by about a degree per hour. (I tested this once when my wife was not home, and it took most of the day to raise the temperature by 8 degrees.)
Because of this, if let the house cool down and then raise the thermostat setting by more than a couple of degrees when we return, “emergency heat” comes on to warm the house quickly. This just uses ordinary heating coils and consumes a lot more electricity than the heat pump would.
In this case, we think it is better to leave the setting alone if we are not going to be away for an extended time. I haven’t tried to quantify this (and I doubt I could persuade my wife to go along with further experimentation).
Note that since there is no such thing as “emergency cooling,” we would be better off letting the house get very warm when we go on a summer vacation, as the physicist would have suggested if that question were asked.
An easy way to prove it is with a watt meter. The kind you plug in the wall and then plug the appliance into the meter. It’ll record how much energy it uses over time. So they just measure how much energy it takes to heat the water from ambient temp, and then measure how much it takes to maintain over a 24 hour period.
@Stephan Bryant
The nice thing about heaters is that the usual definition of efficiency doesn’t apply to them. Normally efficiency is defined in terms of how much energy is used doing useful work vs. how much energy is lost to heat. But a heater doesn’t do work, it just makes heat. “Emergency heat” should be using more power because it’s generating heat faster. I can’t imagine that it’s accidentally doing work; both heaters should be doing the same thing.
The advantage of a heat pump is that it is not using energy directly to “create” heat; it is moving heat from outdoors to indoors.
https://energy.gov/energysaver/heat-pump-systems
According to this article, a heat pump may require only 50% of the input energy compared to a resistance heater. From a short-term energy efficiency standpoint, you want to avoid the “emergency heat” resistance strips coming on. The time involved becomes significant – the energy savings per hour with the heat pump off needs to offset the higher energy use during heatup when “emergency heat” comes on.
Does the same principle applies to storing electric charge?
Should I keep my laptop plugged in except for the times when I need to take it away and use battery, or should I go though empty-full-empty battery cycles?
I am asking strictly from the energy perspective, let’s disregard battery wear in this case.
@george
Batteries do lose charge over time and there is some inefficiency in both charging and discharging a battery, but those are all (remarkably) minimal effects for a “healthy” modern battery. Compared to, say, the loss from sending electricity through power lines, batteries are really good.
@george
Also keep in mind that when you unplug your laptop, the operating system usually puts most of the components into a low power or power saving state, depending on the configuration you have. So if you plan on measuring the electricity usage over time, you need to either take this into consideration or set it so that the components don’t go into this power saving mode. Otherwise, over the course of, say, an hour, it will look like discharging/recharging will use less electricity than keeping the laptop plugged in. It will use less electricity, but not because of battery efficiency. Also pay attention to heat. The hotter your battery, the faster it will discharge.
Keep in mind that wear affects what you should do to maintain a healthy battery, so don’t just worry about efficiency. Keeping a battery fully charged all the time puts stress on the battery, as well as charging and discharging. Modern batteries are supposed to protect against overcharging, but they don’t always. My Samsung Galaxy S5 battery had to be replaced recently because it could not protect itself from overcharging, in fact it was bulging quite a bit. The battery would say it was at 50% one minute, and then the phone would shut off the next.
Thus, practically, you want to do shallow battery discharges to keep it healthy. A deep discharge (making the battery go below ~25ish %) shortens the battery lifetime more than shallow discharges do. But more than anything else, heat will kill your battery life. A cooler battery is a happier battery.
The internal discharge will be faster with more charge. So theoretically, keeping it charged will lose more energy than going through full cycles.
In the first equation of your answer gravy, the 2nd derivative should be WRT x, not t.
@Bf
Thanks for catching that!
“. . . the most efficient thing you can do is keep the temperature as low as possible for as long as possible”.
Well, you can make the temperature as low as possible using a chiller. But that would take energy to operate (and would generate additional waste heat!). When you return you’d have to add back the same amount of heat you removed with the chiller. On top of that there’s the heat you’d have to add had you not used the chiller.
Turn off the heater, yes. Improve insulation, yes. Temp as low as possible, no. Just don’t add heat while you’re away.
“The most energy efficient thing to do is always to turn off the heater.” But there is an exception: when you heat your home with a heat pump. If the temperature goes low enough that you’ll need to turn on the emergency coils when you return, you’ve lost.
Well, this also depends on how long you’ll be away.
I have a different and less sciencey definition of efficiency.
Aside from heat lost and heat retained, there is also heat used. In the case of my fish tank, the heater has a roughly 50% up time when the tank it at the set temp and the heater is just maintaining. However when the tank is at ambient or lower the heater is on roughly 100% of the time till it reaches the set temperature. Also keep in mind the the heat sink is the entire surface area of the water. While the heater is a high temp but low contact area element.
So if your pool takes more than a day and a half to get up to temp before you’re willing to use it, then it was a wast of energy and more importantly money, turning it off for three days.
And yes there’s numbers are guesstimates for a 300 gallon aquarium system, not a 12,000 gallon pool. So actual results may vary.
If your pool is in Miami, there will be no reason to know the answer to this question.
I’ve always been fascinated by the corollary idea that nature makes all this work without math. The diffusion equation is extremely hard to work out to express how what you see cooling with your naked eye is what’s happening.
Airplanes don’t need equations to fly. They just do. Like birds. They don’t need math to flap their wings and fly.
“Not to offend anybody, but it looks like women have better intuition than men.”
It is important to remember that human beings are individual. Trust logic from one only when based on facts. “Logic” can be drawn from all sorts of things, so rather than pick the correct opinions on gender, I would select the one coming to the correct conclusion based on facts. It could be logical to think someone breaking in front of you is at fault for you hitting them (random), or that a paint mixer can also mix thinset (random), but without facts, all you have are opinions of ignorance. Research and post questions (like on this site) and you’ll be so much better informed than just selecting, say someone with brown hair, as your guide.
“Airplanes don’t need equations to fly.”
But we need math to build them so they do, or they wouldn’t. Without an understanding of HOW it works, we can’t make it work. On the other hand, birds can fly, and it doesn’t matter if they understand why. We can walk, and it doesn’t matter if we understand how we do it. But if we were to try to make a robot walk, we definitely need a deep understanding of the physics to create it.
Likewise, I don’t need to know how an engine functions, but someone does, and if I were to build a car, I would have to. Telephone wires were “discovered” at some point to carry sound and then data, now we do it all via cell towers and satellite.
And then on the grander scale of life, at some point, an understanding of physics and math will help us understand more of how the universe functions in way that will allow us to use energy or perhaps “doorways” we haven’t even discovered yet.
I have a question similar to the original post. Same question but what if the pool was an indoor pool with a furnace to maintain ambient air temperature and a HRV system to move air. Since there is very minimal cooling happening to the pool is it better to keep the heater on or turn it off?
JoeyJ,
If you need to apply heat (from whatever source) in order to maintain the temperature of the pool or object, then it is losing heat (through conduction, evaporation, radiation). Therefore, it will cost more energy to keep it warm than to let it cool and warm it up again later. That’s assuming a constant ambient temperature, not talking about waiting for hot weather to warm it for you, like I do with my house (barely heated it last winter, fun little experiment), in which case it can be free.
Bottom line: don’t even heat your pool at all, you need to know that the cooler the better for your health and to be refreshing.
It’s always best to do exactly what you want to do within your budget.
If you have an indoor pool and your ambient temperature is constant, why not have a constant pool temperature. I think the question was about most efficient, but if you have an indoor pool, I might throw the little bit of efficiency out the window for the convenience of having a pool ready for you whenever you feel like swimming.
The same could be said for an outdoor pool, but with an outdoor pool you’re generally paying a LOT more because the temperature is likely to continuously cool the pool down much more. So if you have an outdoor pool, and a few thousand a month, and you want to spend it on keeping a pool ready to swim in, that would be up to the person and their finances.
An indoor pool seems like a much easier, and less costly venture to have that convenience.
This is the most amount of useless conversation i’ve ever read. And the question was never really answered. The question was ,should I turn my heater off every night and start it again in the morning or leave it on continuously. I want to be able to use the pool whenever I want and to have it at my comfort level. It also didn’t include whether it was an above ground pool with steel and vinyl walls, or an inground pool with a thick Masonary wall. A Masonary wall takes great deal of time to heat up. And therefore the difference in heating a Masonary pool as opposed to a vinyl steel liner pool would be completely different. Also a great deal of consideration has to be giving to the average air temperature during the period of heating the pool.
My experience with daytime temperatures in the 80s and nighttime temperatures in the 60s, and having a Masonary pool. It takes a great deal of time for the initial heat up. Therefore keep it always set at 85 , the savings of trying to turn it off, and then start from scratch if you were using it daily or every other day is insignificant.