Physicist: Why bother going out to mine asteroids? Why not stay home? The answer is a little grim. Consider Easter Island, or Iceland, or England; each are isolated places that were once covered in vast forests with abundant animal life and which are now famously barren (those poems about the beauty of the windswept English moors should rightfully be about vast not-windy forests). Now imagine that you and everyone you’d ever known or heard of were living on Easter Island in the waning days of its civilization. There are a few options available: you could stick around and try very hard to cultivate the few remaining resources available, to fix everything before being adventurous, or you could start building boats. Not to leave, but to reap the benefits that come from access to different, unimagined environments, and to dilute the impact you have on your little island. Today, despite still having only a tiny forest and not nearly enough farmland to support it, Easter Island has a larger population and more infrastructure than ever before in its history.
For those of you reading between the lines, Earth is an island too. But unlike Easter Island, we have to do absolutely everything “in house” and effectively no access to the outside world. Everything humanity does directly impacts the Earth and, while that sounds like a simple truism, it doesn’t have to be. Earth is tiny compared to the sum of planets, moons, and smaller bodies in the solar system. While Earth has a lot going for it, it’s about the worst possible place to look for a lot of the resources that make our civilizations possible and the absolute bottom of the list for places to be cavalier with pollution.
The oldest known examples of iron tools showed up during the bronze age, before the invention of iron smelting. When Tutankhamun died 34 centuries ago he was buried with an iron dagger, which is noteworthy for a couple reasons. First, iron smelting (turning ore into iron) is complicated and energy intensive, which is why Egyptians didn’t start doing it until 8 centuries later. It may have come from somewhere else and been given to the child king as a gift, but that wouldn’t explain the dagger’s peculiar alloy: a literally other-worldly 11% nickel impurity.

Left: Tutankhamun’s incredibly rare and valuable iron knife with it’s cheap-as-dirt gold sheath. Right: A metallic meteorite. The pretty lines in the cut are “Widmanstätten patterns”, which form when iron and nickel are allowed to cool slowly over millions of years.
Metal working was invented before smelting, which is fine for gold since “metallic gold” exists on Earth. You can literally dig it out of the ground, dust it off, and start making jewelry. But metallic iron doesn’t naturally exist on Earth. After billions of years stewing in our water and oxygen atmosphere, any pure iron that might have existed has rusted away and combined with rocks and minerals long before any person could have found it. So it’s not a coincidence that the oldest worked iron show clear signs of being from space.
In fact, using a metal detector is the quickest way to find space rocks (were you so inclined). Of course, Earth is a giant space rock made of lots of smaller space rocks, so you’d think that Earth would be just as metal rich as asteroids. And you’d be right. Unfortunately, Earth is mostly molten. At one time even the surface was mostly molten and since then, through plate tectonics, huge chunks of the crust get remelted every few hundred million years. That gives the heaviest material plenty of opportnity to separate out and sink, like dirt settling out of water. So Earth does have a heck of a lot of heavy metals, but almost all of it is stuck in the core, starting at 3,oo0 km below your feet. So sure, there’s a huge amount of iron, uranium, and other heavy metals in Earth, but it doesn’t really count. Not for us anyway. All of it is going to stay right where it is.
The same gravity that makes Earth one big rock instead of billions of small rocks makes life on Earth both possible and difficult. Without gravity we wouldn’t have air to breathe, or oceans, or any of our favorite not-tied-down stuff. But it does make life worse. Ask yourself some very basic questions about mining: Why is it so hard to dig a hole and keep it from collapsing? Why does it take so much work to get stuff out of that hole and get it where it’s supposed to go? Gravity.

It takes a shocking amount of energy to pulverize everything in this city-sized hole, pull it to the surface, and move it overland to somewhere else. And that’s step one.
In short, Earth is a hellscape of corrosion and gravity. We live in about the worst possible location for mining. The Moon isn’t ideal either; it’s also stratified with most of its metals in its core, but at least there’s no air. Small blessings.
We’ve already mined all of the high-purity “low-hanging fruit” resources that we can find (Technically, the best iron and nickel mines are asteroid mines, they’re just ex-asteroids). Every element has its own story and processing requirements, but in a nutshell, when you mine for something you’re digging up rocks that include what you’re looking for as just a small fraction of their chemical makeup. For example, to get aluminum we generally dig up bauxite which is composed of aluminum, oxygen, hydrogen, and a bit of iron. To remove the aluminum we have to break bauxite down to its atoms and chemically sort them to get aluminum by itself. Some processes are more efficient than others, but at the end of the day there’s a cost to pay to the gods of entropy when you sort anything into its base elements. Three buckets with aluminum, oxygen, and hydrogen have much lower entropy than a pile of bauxite, so there’s a thermodynamic limit to how good the process can be. It will always reqire lots of work. Pointedly for us (and everything else that lives), between the massive energy consumption, incidental poisonous by-products, and direct damage to the land, large-scale mining and industry is really, really not the sort of thing that should be done on Earth.
What we have access to here on Earth is a pittance and gathering up that pittance means scouring the surface of the Earth, which is not ideal. When someone says “there are resources in space” it’s more accurate to say “all of the resources are in space”. Heck, there’s more than twice as much liquid water on Europa than Earth, so we don’t even have the water market cornered. In addition to its millions of smaller cousins, the asteroid belt hosts the exposed metallic core of a shattered protoplanet, 16-Psyche (named using a very old convention: it was the 16th asteroid discovered). It’s over 200 km across, the size of a state, made almost entirely of heavy metals, and could satisfy just our current iron consumption (about 1.2 billion tonnes per year) for about 18 million years. With a surface gravity of about 1.5% of Earth’s, a child could lift and throw a car’s worth of mass, so moving material around is literally child’s play. So that’s a practically infinite supply of easily mined construction materials, nuclear fuels, and the increasingly rare elements we need for our increasingly fancy technology, in a form that’s more pure than we’ll find anywhere on Earth, and all in a place where we couldn’t destroy the environment if we tried. And 16-Psyche is just 1% of the mass of the asteroid belt. It’s a good place to start.
Admittedly, the asteroid belt is a long way away. But distance isn’t the problem. Getting around in space is embarrassingly easy; it’s leaving Earth that’s a pain. When we think of space travel, we usually imagine huge, and more importantly expensive, rockets. Earth, it unfortunately happens, is the hardest place in the solar system to leave (there are bigger planets, but they don’t have solid surfaces to leave from). To achieve low Earth orbit you need to accelerate to 8 km/s, almost 24 times the speed of sound, and all of that accelerating needs to be done in just a few minutes. Going half-way to space means being back on the ground in short order.
Jumping that lots-of-acceleration-right-now hurdle is the job of “launch vehicles”, which are always chemical rockets. Despite all the glamour and showmanship, chemical rockets are primitive like steam engines are primitive. The Apollo missions were sent to the Moon on a mix of refined kerosene and liquid oxygen; the sort of fuel you might throw together if your camp fire was boring. The exhaust from that burning kerosene explodes out of the rocket bell at 2.5 km/s, which could stand to be a lot faster. Unfortunately, if you need high-thrust right now, there aren’t presently any other options. NASA made up for the sluggishness of their propellant by using a lot more of it.
But once you’re in space, you can take your time and use ion drives. They’re not as clumsy or random as a rocket; an elegant engine for a more civilized age. Ion drives fire off their propellant on the order of ten times faster than chemical rockets and that’s a really big deal.
When you use propellant, you’re not just moving your rocket, but the propellant you’ll be using later. So if you can use that propellant more efficiently, by throwing every bit of it as fast as possible, then you not only need less it to move your spacecraft, you need less propellant to move less propellant. The gain in efficiency is exponential as the rocket equation makes explicit; the ratio of the spacecraft-and-fuel’s initial to final masses is , where ΔV, “delta vee”, is the change in speed the rocket is capable of and Ve is the velocity of whatever the rocket is throwing out the back.
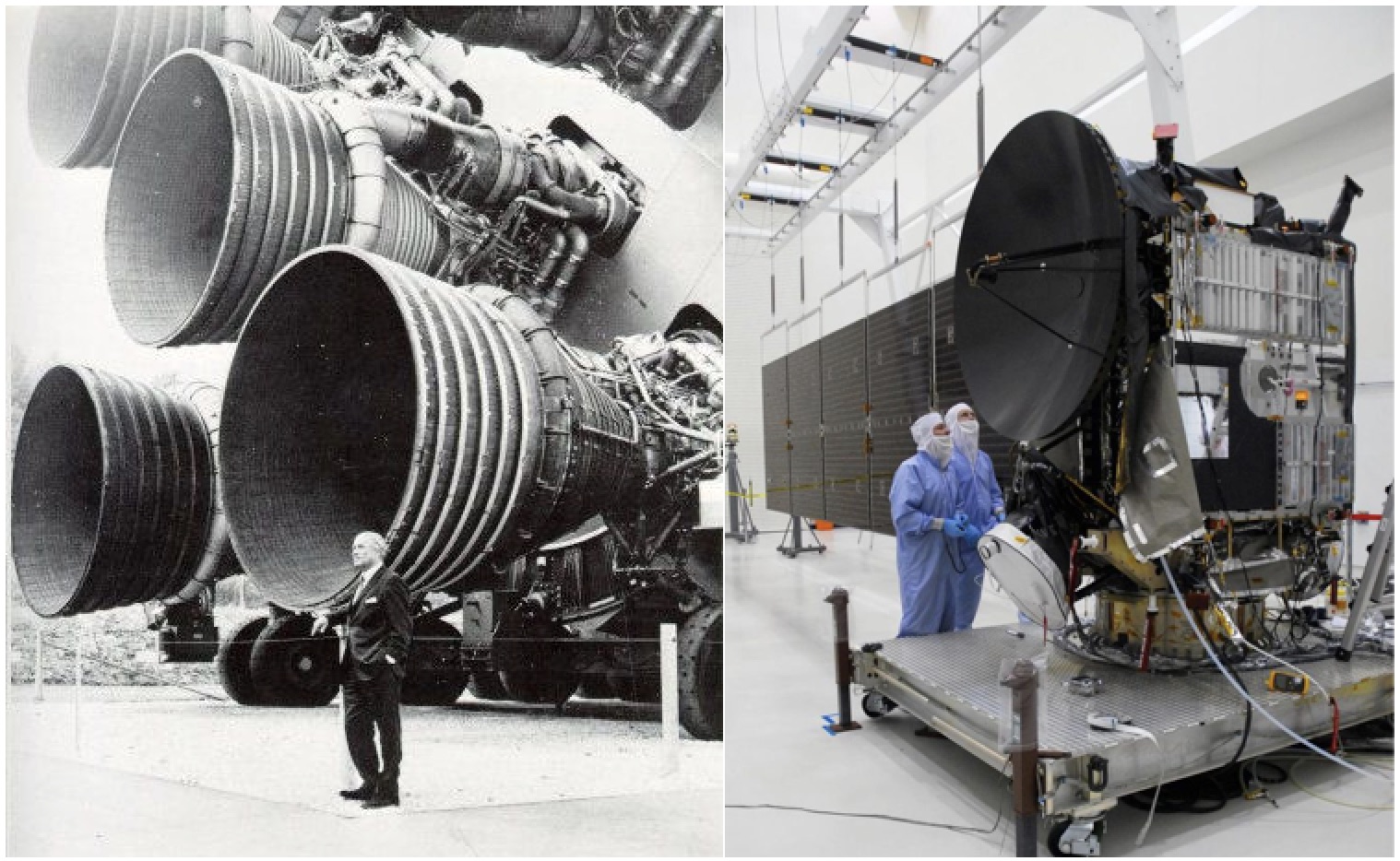
Left: Von Braun next to one of the chemical rocket engines of the Saturn V, a launch vehicle. Right: Faceless engineers next to the Dawn probe, a spacecraft. Over eleven years, Dawn’s solar powered ion drive used less than half a ton of xenon to accelerate a total of 11 km/s as it investigated multiple targets in the asteroid belt. That’s faster than the Saturn V.
The Dawn spacecraft traveled to and orbited both Vesta and Ceres, the second and first largest asteroids in the belt. Being the first to do it makes Dawn a first-generation rock-hopping spacecraft. We can reasonably expect the spacecraft that will eventually move around in the belt will be at least this capable.
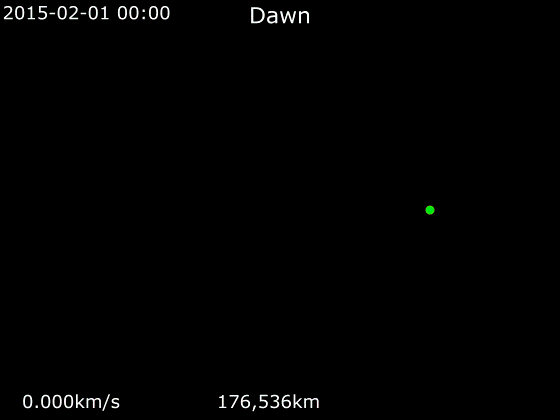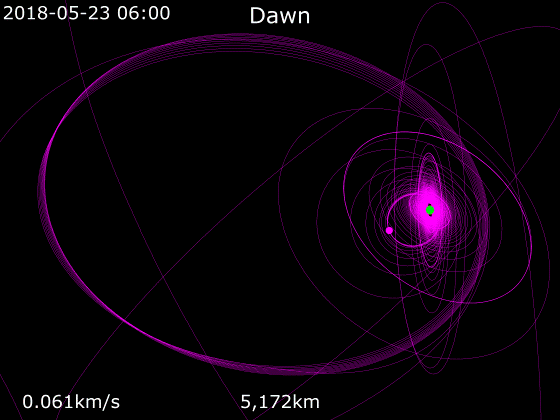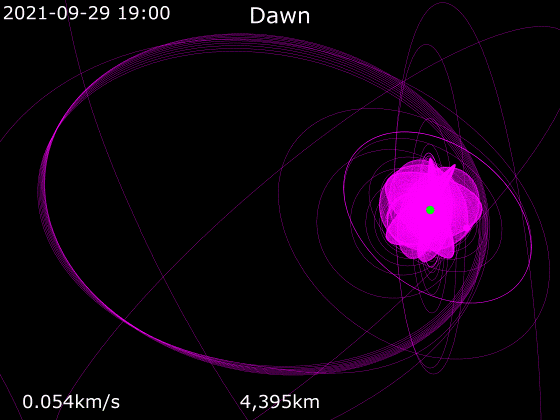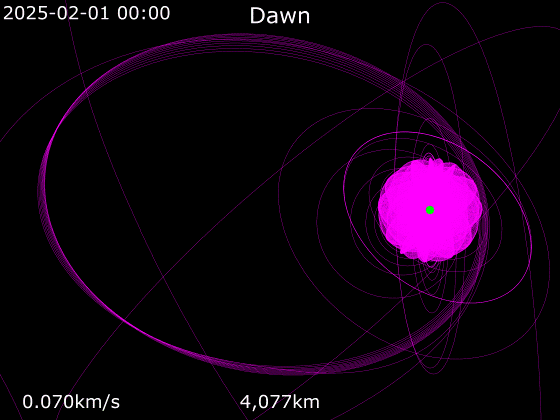
What Dawn did, with its fancy-dance ion drive, when it got to Ceres. It’s clearly not 2025 yet, but it ran out of fuel two years ago (Halloween 2018), so we can be reasonably confident about where it will be in the future.
Being able to use ion drives and gravitational slingshots spells the difference between compact, efficient, long range “spacecraft” and bloated, wasteful “launch vehicles”. Traveling between planets and across the solar system is genuinely much easier, safer, and eventually cheaper than clawing your way into low Earth orbit.
That “eventually” is the problem and a solution. If we can build and refuel spacecraft off-Earth, then we’ll be able to pump out cheap, truck-sized interplanetary spacecraft capable of moving a few tons of cargo anywhere in the solar system (if you’re patient). On Earth there’s an engineering limit to how big you can build because of our ancient nemesis gravity, but once you’re building in space, size ceases to be a concern; we could build and efficiently slingshot entire cities around the solar system if we felt like it. Building a huge launch vehicle to leave Earth, mine an asteroid, and return is a terrible way to get resources back to Earth. Building thousands of cheap, tiny spacecraft on the Moon to travel to and from any of dozens of established mining colonies on 16-Psyche is also expensive, but much more efficient with much greater returns. But manufacturing requires infrastructure, which means that a lot of what you recover in mining will be used to support the in-situ infrastructure that supports the mining. And the people involved. And their families. And sooner or later (probably sooner) their legal issues.
Here’s a terrible metaphor. If Henry VII (the king of England during the European realization of the New World) had wanted to mine gold in California, he would have had to fund a Lewis-and-Clark type expedition with all the necessary equipment and extra personnel to cross the continent, and put all of that onto a fleet of ships to get to the east coast. It would have been a losing proposition. But today, with plenty of intervening infrastructure, Elizabeth II could pack her corgis, trace out the same route as that hypothetical expedition on a boat, then a train, then a cab, buy a shovel when she arrives, and be in the gold business in no time. It feels like building the United States is an inefficient way for English monarchs to mine gold, but keep in mind that mining gold isn’t entirely the point. It turned out to be good for England (In retrospect. Arguably.) in ways that they couldn’t possibly have predicted.
Expanding into space means creating new nations, new industries, and new places to go. There are a lot of advantages to an essentially infinite supply of dirt-cheap resources and power. More importantly, it means giving Earth a chance to lick its wounds and recover from what we’ve done so far. We, as a species, have a hunger for energy and a taste for the rarer elements in the universe. We’re not going to stop hunting for resources and building things, but we don’t have to do it here.
Asteroid mining isn’t about someone somewhere making trillions of dollars, although that will probably happen. It’s not about advancing science, although that will definitely happen. It’s about gaining access to the vast, vast majority of useful materials and fuel in the solar system, while making Earth-based mining and industry expensive and filthy by comparison.









After the dawn graphic, 4th line, second word “a” should be “an” because the next word, “engineering” starts with a vowel. Sorry to bust your balls about this.
@AHelperOfGrammarAndSpelling
Fixed!
I truly appreciate it. Not sounding dumb is a uphill battle.
Beautifully and entertainingly put.
Thank you!
How terribly interesting!
And so well written! It’s a pleasure to read.
Thank you again for the time and effort you lot (whoever you are) put into writing these articles.
Primitive animals consumed what was around. More advanced animals began active search for food. Even more advanced animals began studying their close surroundings, and this gave them advantage: they knew what is around and what could happen. Even more advanced animals (humans) began studying not just their surroundings, but everything, and got an advantage, because future is unknown and studying everything can be helpful if environment or situation changes. So studying everything is advantageous over studying just specific things.
That’s why by the way, the scientific society has a greater advantage over the religious one; whilst traditional societies keep unchanged for centuries they suddenly see airplanes and spaceships flying over their heads.
But what do you need it for? Consider this scenario – that is from my sci-fi novel, Writings in Science a History of the Future.
The population reduces drastically. There is too much of everything for those left. Houses are free – just pick an empty one. Antiques and furniture now built to last for almost ever, clog every city, take what you want. Virtually everything made is smaller and smaller in size. Greed is not an issue, want is not an issue, having anything is not an issue. Having too much of everything and conserving much of it, is! Manufacturing and farming is either in house, in the city/states, or there are 3 major manufacturing centers – 2 on Earth, one on the Moon – that manufacture all big things. Virtually everything is recycled, etc. So who exactly are you mining for?
I apologise Physicist. I don’t want to be polemic but only to understand.
A Italian scholar wrote: “can extract minerals on Earth because of the “energy credit” that comes from geological or biological processes (and often both) which have concentrated specific elements in some special regions of the crust. We call these regions “deposits” and we use the term “ores” for those deposits which are concentrated and pure enough that they can generate an economic profit from mining. Only ores are a useful source of minerals. Mining from the undifferentiated crust is simply unthinkable because of the enormous energy it would require”
And: “The physical processes that created ores on our planet can take place only on planets which are both geologically and biologically active. As far as we know, asteroids never were. So, there are no ores on asteroids; nor there are on the moon or other “dead” space bodies.”
So, i don’t understand. Are you right? Is he right? Or what else? Thank you very much
@Robo
Nothing to apologize for! Please doubt me.
There are a lot of physical processes that concentrate one thing or another. The Cliffs of Dover have a lot of calcium because they’re millions of years of sea creature shells. The Oklo Mine uranium mine was made by a river eroding uranium from rocks upstream and allowing it to settle out in a particular section of the river.
But there aren’t any processes on the surface of Earth that come close purifying most heavy metals (gold and silver are among the only exceptions). In fact, “rare Earth metals” aren’t necessarily rare, just spread out.
Iron on the surface of the Earth does get concentrated, but even the best locations are less than 40% iron by weight. But the same process that concentrates heavy metals in the Earth’s core works in our favor in space. Just form a small planet, big enough to be molten, let it stratify, then smash it apart with impacts. That’s likely to be where “M-type asteroids” come from. This seems to have happened at least a few times. 16-Psyche is a great example; more than 90% iron and nickel.
How DID Tutankhamun get that knife then?
@Gol
1) Find a metallic meteorite that’s fallen to Earth.
2) Smith it into the shape of a knife.
3) Give it to your child god-king.
In all likelihood, Tut’s knife was a piece of something much larger, like the Cape York meteorite, since it’s unlikely that someone would say “Hey, look at this small weird rock made of a material I’ve never seen! Guess I’d better make a knife and give it to my god-king!” Bigger samples attract more attention from bigger groups of people and give them an opportunity to practice.
@Robo
I too am guilty of unnecessarily complicating simple things.
The Italian scholar, defining “ores” and “deposits”, and getting stuck in the minutiae remind me of myself.
But there is a forest inclusive of those trees.
All of what you said describes the difficulty (and value) of finding mineral-rich concentrations of described elements in the crust of the Earth, an ancient planet with countless other types of “stuff” that dilute the less plentiful ingredients. Silica (sand) and clay and granite and entropy all conspiring over billions of years to make the “ores” comparatively rare and their “deposits” varied in economic value to recover. Yet these same geological and biological forces which provide the “energy credit” for creating deposits of ore are also, entropically speaking, responsible for making the bulk of those elements in the crust so scattered, creating for the most part, zero recoverable value.
On a dead planet, eventually all of those heavy ores would indeed disappear from the crust and be unavailable due to being out of reach at the core. Which is exactly why the asteroid 16-Psyche is made of such a high concentration of them: It was almost a planet but had all that “stuff” ripped away, leaving just the heavy metals concentrated and exposed for easy access, a floating planet core.
Did the Italian scholar happen to mention anything about being lucky?
@The Physicist
Perhaps ancient aliens gave it to him?
@Gol
Unlikely, it shows all the typical signs of human make. If aliens made the knife we would expect it to be the product of anomalous forging methods, instead it appears to be made from a chunk of metallic meteorite that was then forged into a knife.
In an older post (I don’t remember which one) you had said “…there’s ion drives but a mouse fart is more powerful, so meh”. Or something along those lines. What’s changed that we are already orbiting asteroids with them?
@Jeff
Because space.
Once you’re in space proper you are free of the confines of friction, this means that even mouse farts can create a measurable and permanent change in velocity. Not much of one, but if the mouse keeps farting every second of every day for years, it adds up.
Ion drives are totally worthless in-atmosphere, but once you get outside of atmosphere they become by far the most efficient method of propulsion currently known. Still quite slow; it takes years if not decades to get anywhere with an ion drive, but robots don’t give a shit about travel times; you can just send them off into space and check back years later when they eventually arrive wherever they were supposed to be going.
As long as you start off outside of a planet’s atmosphere, ion drives can take you to anywhere in the solar system at a tiny fraction of the fuel cost needed for any other known form of propulsion. Considering that fuel costs are exponential, due to needing fuel to move the fuel, needing orders of magnitude less fuel to get somewhere is a Very Big Deal.
@Jeff
Human fart. That’s still more or less the same.
Rockets are very impressive, but they only fire for a few minutes. Ion drives fire continuously for months, giving that tiny force a chance to add up to a lot.
Let’s say an ion drive and a Saturn V were to do a drag race, both starting their engines at the same time. How long would it take the ion drive to catch the Saturn V and how far away would they be from the start when the the pass was made?