Something IBM made with some very flat, very clean, very cold copper and a few hundred carbon monoxide molecules.
Physicist: Actual pictures of atoms aren’t actually pictures at all.
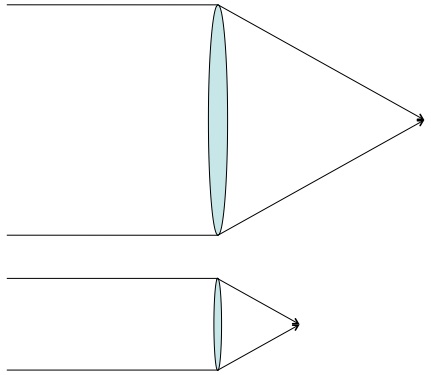
There are a few good rules of thumb in physics. Among the best is: light acts like you’d expect on scales well above its wavelength and acts weird on scales below. In order to take a picture of a thing you need light to bounce off of it in a reasonable way and travel in straight lines (basically: behave like you’d expect). But the wavelength of visible light is about half a micrometer (a two-millionth of a meter) and atoms are around one ångström (a ten-billionth of a meter) across. On the scale of atoms, visible light acts too wonky to be used for photographs.
Atoms are literally too small to see.

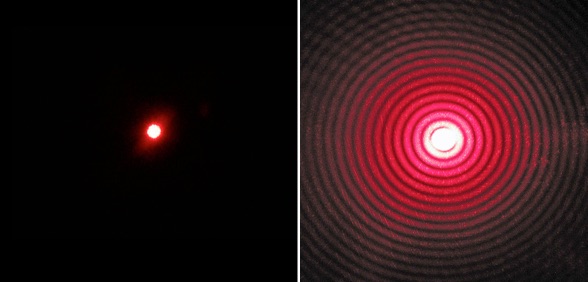
(Left) A photograph of a 3 ball. (Right) What we have in lieu of a photograph of an atom.
You could try using light with a shorter wavelength, but there are issues with that as well. When light has a wavelength much shorter than an atom is wide, it takes the form of gamma rays and each photon packs enough energy to send atoms flying and/or strip them of their electrons (it is this characteristic that makes gamma rays dangerous). Using light to image atoms is like trying to get a good look at a bird’s nest by bouncing cannonballs off it.
There are “cheats” that allow us to use light to see the tiny. When the scales are so small that light behaves more like a wave than a particle, then we just use its wave properties (what else can you do?). If you get a heck of a lot of identical copies of a thing and arrange them into some kind of repeating structure, then the structure as a whole will have a very particular way of interacting with waves. Carefully prepared light waves that pass through these regular structures create predictable interference patterns that can be projected onto a screen. Using this technique we learned a lot about DNA and crystals and all kinds of stuff. This is the closest thing to a photograph of an atom that is possible using light and, it’s fair to say, it’s not really what anyone means by “photograph”. It’s less what-the-thing-looks-like and more blurry-rorschach-that-is-useful-to-scientists. Even worse, it’s not really a picture of actual individual atoms, it’s information about a repeating structure of atoms that happens to take the form of an image.
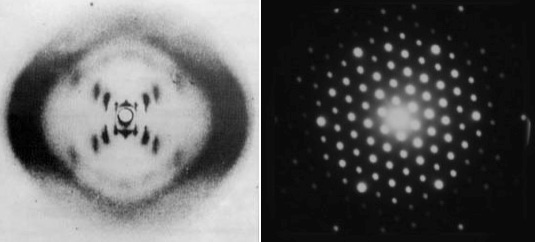
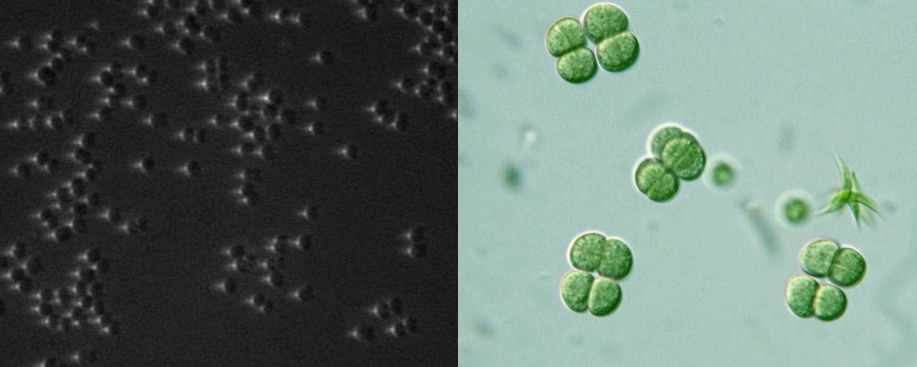
By passing light (left) or even electron streams (right) through a regular, crystalline structure we create an interference pattern that gives us information about the structure of the crystal (but never pictures of individual atoms). The picture on the left (left) is a pattern created by DNA (species unimportant). Notice how not obvious the helical structure is. The picture on the right is created by an electron beam passing through some simple mineral or salt.
These techniques are still in use today (are relatively cheap), but since 1981 we’ve also had access to the Scanning Tunneling Electron Microscope (STM). However, despite the images it creates, the STM isn’t taking a photograph either. The STM sees the world the way a blind person on the end of a tiny robotic arm sees the world.

The essential philosophy behind the Scanning Tunneling Electron Microscope is what allows this dude to know more about the bottom of this chili cauldron than you do.
The STM is basically a needle with a point that is a single atom (literally, it is the pointiest thing possible) which it uses to measure subtle electrical variations (such as a stray atom sitting on what was otherwise a very flat, clean surface). The “Tunneling Electron” bit of the name refers to the nature of the electrical interaction being used to detect the presence of atoms; when the tip is brought close to an atom electrons will quantum tunnel between them and the exchange of electrons is a detectable as a current. The “Scanning” bit of the name refers to how this is used to generate a picture: by scanning back and forth across a surface over and over until you’ve bumped every atom with your needle several times. The pictures so generated aren’t photographs, they’re maps of what the STM’s needle experienced as it was moved over the surface. The STM “sees” atoms using this needle in the same way you can “see” the bottom of a muddy river with a pokin’ stick.
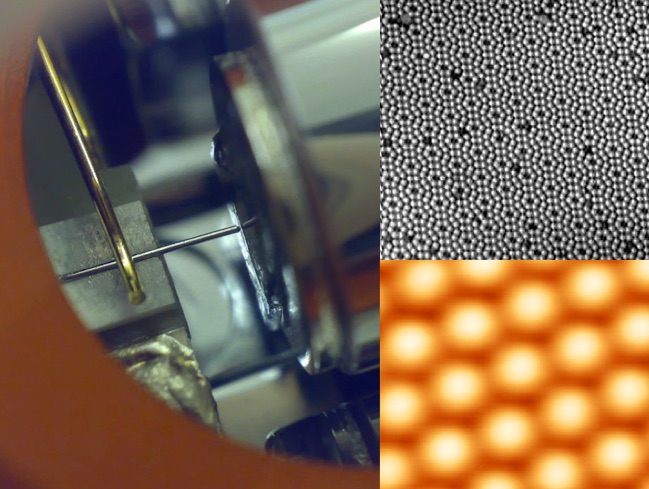
An STM and some of the pictures it pokes into being.
This technology has been around for decades and, like the advent of the synth, has given rise to all manner of jackassery.