Physicist: Thanks to a comment in the original post, I did a little research and found that I was wrong, wrong, holy crap wrong. Here’s some of that comment:
“The source of the ultimate “excluded volume” forces is entirely quantum mechanical: it is the fact that electrons are fermions, hence (this is the Pauli exclusion principle) cannot occupy the same volume without being in different energy states. If you attempt to push the orbital electrons of two atoms into the same volume of space, most of the electrons will need to be promoted to much higher energy states. The requirement of a great deal of energy to move the atoms closer is what we interpret as a force. You might call it an “exclusion” force, because it comes from the exclusion principle —”
One of the nice properties to come out of the depths of quantum physics is the “Pauli Exclusion Principle“. “Nice”, because it can be understood without a couple years of extra schooling. Essentially, two “fermions” (which is a classification of particles that includes all ordinary matter: neutrons, protons, and electrons) can’t be in the same state. That state includes (in the case of electrons in atoms) electron orbitals.
You could argue (successfully) that the Pauli exclusion principle is responsible for all of the complex chemistry of the universe. If electrons didn’t “stack up” into higher and more complicated shells, then every element would have more or less the same chemical properties as hydrogen and helium. Instead, as you move along the periodic table, every new electron tries to settle into the lowest possible state, but finds that many of them are occupied. So, it ends up in a high energy state, because it can’t find any that are lower.
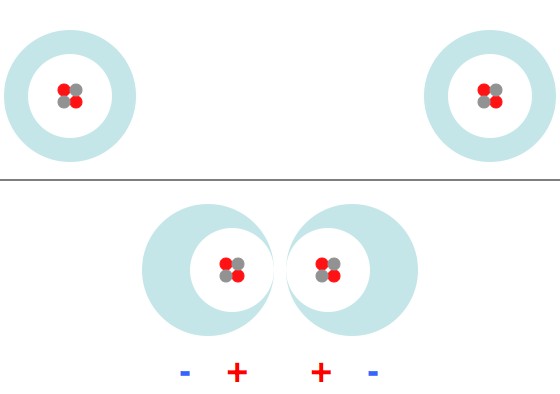
When two atoms are brought very close together their electron orbitals start to overlap and the atoms start to “share” electron orbitals. In some cases this takes the form of a chemical bond (and the atoms get stuck together). More often sharing an orbital means that the electrons already present are forced into higher energy orbitals. By necessity, the effect kicks in when the atoms are close enough that you can’t tell which electron came from which atom (for a few electrons at least).
A good way to define a force is a system’s attempt to get rid of energy (specifically: , which is just fancy speak for “stuff rolls downhill”). If the atoms are too close together some electrons find themselves in high-energy states, and if the atoms are a little farther apart those electrons will be in lower energy states. So, there’s a force that pushes the atoms away from each other, once they get very close together.
So, the repulsion effect isn’t directly caused by electric forces, it’s more of a side effect. But, if you could somehow “turn up” the strength of the electromagnetic force in our universe, you’d find that the repulsive effect increased in kind, because the amount of energy tied up in the electron’s energy levels is proportional to the strength of the electromagnetic force.
That’s a bit subtle. It’s a little like saying that when you push a car up a hill (while the car is trying to push you back down the hill), you’re not fighting gravity directly, you’re fighting the effect of gravity on the car.